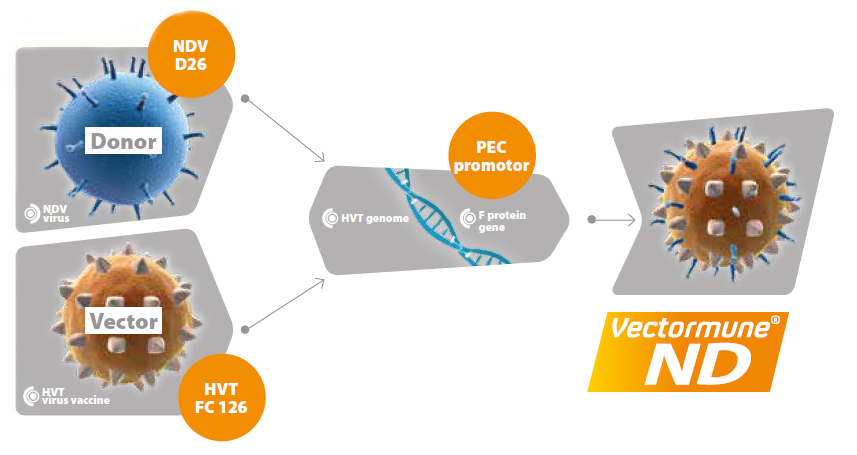
VECTORMUNE® ND contains a live frozen serotype 3 Marek (HVT) vector vaccine virus indicated for use in chicken. The HVT in Vectormune® ND expresses key protective Newcastle vaccine virus. It is indicated as an aid in the prevention of Newcastle disease and Marek's disease in chicken.
Composition:
Frozen minimum titer of HVT-NDV is 3420 PFUs
Indication:
Recommended for use in day-old chicks or in 18 to 10 day-old embryonated chicken eggs as an aid in the prevention of Newcastle disease or day-old chicks
Dosage:
Day-old chicks: 0.2 mL subcutaneous injection
In-ovo: 0.1 mL/0.05 mL
Recommended age of vaccination:
Day-old chicks OR
18-19 day-old chick embryos
Precaution:
Do not take out of liquid nitrogen until ready to use.
Do not vaccinate within 21 days before slaughter.
Do not re-freeze vaccine.
Burn containers and all unused vaccines.
Withdrawal Period:
21 days
Presentation:
Vial - 2 mL in dewars with liquid nitrogen
VECTORMUNE® ND (HERPES VIRUS TURKEY-NEWCASTLE DISEASE VECTOR)
Vectormune® ND is a recombinant HVT vector vaccine, in which the genome the "F" gene of a genotype I NDV has been inserted. The HVT strain used (FC 126), its origin, the low number of passages applied, the "F" insert, the insertion site, the promoter selected to ensure the expression of the F gene, the terminal sequence, etc... are all key elements explaining the uniqueness and outstanding features of this vaccine. Most of these features have been patented and belong to Ceva Animal Health. Vectormune® ND is unique and cannot be confused with other rHVT-ND vaccines.
The "F" (for "fusion") protein is the epitope present on the surface of NDV, allowing it to attach and penetrate target cells. It is, at the same time, a key factor of virulence of the virus, as well as a key protective antigen. One can easily understand that if immunity is built up against "F", then NDV would have much more difficulty infecting cells and creating damage, which probably explains the very high efficacy of Vectormune® ND. Vaccinated chicken are protected against clinical and economic consequences of NDV infection - the replication of NDV inside the chicken's body is limited as indicated by a reduction in shedding of the challenge virus as well as higher antibody titers following infection.
The HVT strain used to carry and express the "F" gene is known for decades as a very safe and very stable virus. It is used worldwide to vaccinate chicken against Marek's disease (MD). The particular strain and passage level used for the construction of Vectormune® ND replicates actively in the chicken and this explains why protection against NDV appears so quickly.
Onset of Immunity
An antibody response to Vectormune® ND (of the type IgG, IgM and IgA) can be detected in SPF chicken as early as 9-12 days post-vaccination (Rauw, et al., 2012).
The immune response to Vectormune® ND is not only composed of circulating antibodies but also of a local immune response. It is not only humoral but also cellular (Rauw, et al., 2010). Antibody response to Vectormune® ND can be detected using the Haemagglutination Inhibition (HI) test, or ND "F" ELISA tests. Following vaccination at day 1, with the presence of passive immunity, antibody response can clearly be differentiated from the controls at around 211-28 days of age. On the reverse, this detection is not possible with commercial ND ELISA kits (e.g., Idexx, Biocheck). If no live vaccine is used, this can be a simple serological method to differentiate vaccinated from infected (commonly known as the DIVA procedure).
Duration of Immunity
Duration of immunity is probably the most impressive feature of this vaccine.
Following a single injection of Vectormune® ND on day of hatch, layers are totally protected against clinical signs, mortality and drop in egg production until, at least, 72 weeks of age (Palya et al., 2012). This cannot be compared to any existing ND vaccination conventional program where a minimum of 2-4 killed and 5-8 live vaccinations would be necessary to achieve acceptable (but not comparable) level of protection.
Vaccination Strategy
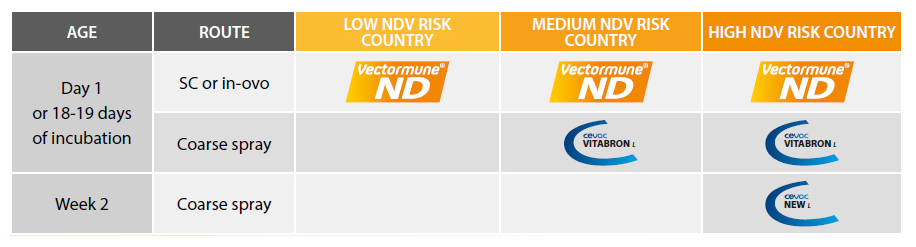
Since protection with Vectormune® ND requires replication of the NVT vector and this generally takes some days, the first 3 weeks of life are gradually covered by Vectormune® ND. For this reason, in ND endemic countries, the vaccination strategy with Vectormune® ND requires ensuring early protection by application of a live attenuated ND vaccine by spray on the first day of age in the hatchery. In order to avoid damages to the trachea, which are detrimental to both growth and integrity of the respiratory tract, it is strongly recommended to use a vaccine based on an apathogenic enterotropic NDV strain, like the Phy.LMV.42 NDV strain present in Cevac® Vitapest L (ND only) or Cevac® Vitabron L (combination of ND + IB).
In countries where ND is only an epizootic risk, it is advisable to remove any live ND vaccine from the program. the combination of a reliable ND passive immunity together with Vectormune® ND-induced active immunity will ensure a very significant level of protection. By removing the use of any live ND vaccine in chicken flocks, this innovative approach helps to improve the overall respiratory health, which could in turn result in less antibiotic medication and lower rates of airsacculitis at the processing plant.
Following several years of investigation with Vectormune® ND, we believe that this vaccine is more a revolution than a simple evolution.
Vectormune® ND has been changing the approach of Newcastle disease prevention in the field and is being considered as a strong tool towards the long term control of this important poultry disease.
Spectrum of protection
Vectormune® ND provides a broad spectrum of efficacy.
Perfect protection has been demonstrated against challenges conducted with high doses of various NDV strains belonging to diverse genotypes, including gentorype II (Texas GB strain, B1B1 strain), genotype IV (Herts 33 strain) genotype V (Mexican Chimalhuacan strain), and genotype VII (several isolates).

 Corporate Website
Corporate Website
 Africa
Africa
 Argentina
Argentina
 Asia
Asia
 Australia
Australia
 Belgium
Belgium
 Brazil
Brazil
 Bulgaria
Bulgaria
 Canada (EN)
Canada (EN)
 Chile
Chile
 China
China
 Colombia
Colombia
 Denmark
Denmark
 Egypt
Egypt
 France
France
 Germany
Germany
 Greece
Greece
 Hungary
Hungary
 Indonesia
Indonesia
 Italia
Italia
 India
India
 Japan
Japan
 Korea
Korea
 Malaysia
Malaysia
 Mexico
Mexico
 Middle East
Middle East
 Netherlands
Netherlands
 Peru
Peru
 Philippines
Philippines
 Poland
Poland
 Portugal
Portugal
 Romania
Romania
 Russia
Russia
 South Africa
South Africa
 Spain
Spain
 Sweden
Sweden
 Thailand
Thailand
 Tunisia
Tunisia
 Turkey
Turkey
 Ukraine
Ukraine
 United Kingdom
United Kingdom
 USA
USA
 Vietnam
Vietnam